When you pick up a generic pill at the pharmacy, you expect it to work just like the brand-name version. But how does the FDA make sure it actually does? The answer lies in bioequivalence-a strict scientific standard that bridges the gap between brand-name drugs and their cheaper copies. It’s not about matching ingredients by weight. It’s about proving the body absorbs and uses the drug in the exact same way. Without this, generics could be ineffective-or worse, dangerous.
What Bioequivalence Really Means
Bioequivalence isn’t a vague promise. It’s a measurable, data-driven rule defined by the FDA in 21 CFR § 320.1. Simply put, two drugs are bioequivalent if they deliver the same active ingredient to your bloodstream at the same rate and to the same extent. That means your body sees the same drug, at the same time, in the same amount-no matter if it’s made by Pfizer or a generic lab in India.
Think of it like pouring two different brands of soda into identical glasses. You don’t care if the bottles look different. You care that the liquid inside gives you the same fizz, the same sweetness, the same effect. Bioequivalence is the scientific version of that test-but for drugs.
How the FDA Tests for Bioequivalence
The FDA doesn’t rely on guesswork. It uses real human trials, called pharmacokinetic (PK) studies. These involve 24 to 36 healthy volunteers who take both the brand-name drug and the generic version, usually in a randomized crossover design-meaning half take the brand first, then the generic; the other half do the reverse. This eliminates individual differences in metabolism from skewing the results.
Two key numbers are measured:
- Cmax: The highest concentration of the drug in the blood after dosing. This tells you how fast the drug gets absorbed.
- AUC: The total amount of drug absorbed over time. This tells you how much of the drug actually enters your system.
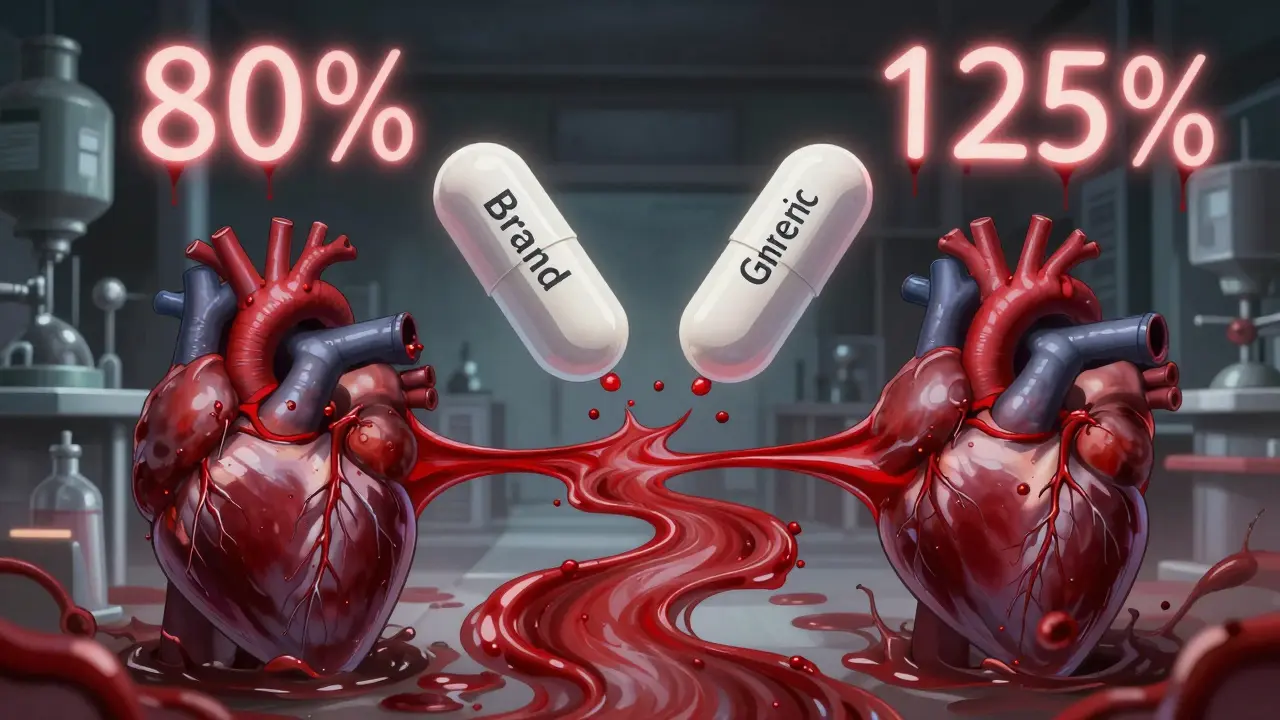
For a generic drug to pass, the 90% confidence interval of the ratio (generic/reference) for both Cmax and AUC must fall between 80% and 125%. That’s not a range of possible active ingredient amounts-it’s a statistical window for how the body handles the drug.
Here’s what that looks like in practice:
- If the brand-name drug has an AUC of 100 units, the generic must show an AUC between 80 and 125 units.
- If the generic’s average AUC is 93, but its 90% confidence interval stretches from 75 to 110, it fails-because 75 is below 80.
- If the average is 116, but the confidence interval is 103 to 130, it also fails-because 130 exceeds 125.
The FDA requires this level of precision because even small differences in absorption can matter. For example, a drug that’s barely absorbed might not control seizures. A drug that’s absorbed too fast could cause dizziness or overdose.
Myth: Generics Can Contain 80% to 125% of the Active Ingredient
This is one of the most common misunderstandings. No, the FDA does not allow generic drugs to contain anywhere from 80% to 125% of the active ingredient. That would be dangerous-and illegal.
The 80-125% rule applies to how your body processes the drug (Cmax and AUC), not the amount of active ingredient in the tablet. The actual active ingredient in a generic must match the brand-name drug exactly, within very tight manufacturing tolerances-usually ±5% or less.
Think of it like this: Two cars might have identical engines (same active ingredient), but one has a better fuel injector (formulation). One might burn fuel faster (higher Cmax), or use more fuel over a long drive (higher AUC). Bioequivalence testing checks if the *performance* is the same-not just if the engine parts are the same.
Why This Matters for Patients
Over 90% of prescriptions in the U.S. are filled with generics. They save the healthcare system an estimated $1.7 trillion every decade. But none of that matters if the drugs don’t work the same way.
The FDA’s bioequivalence standard ensures that:
- A generic blood pressure pill won’t suddenly spike your heart rate.
- A generic antidepressant won’t cause withdrawal symptoms because your body isn’t getting the same steady dose.
- A generic seizure medication won’t let a patient slip into a dangerous episode.
For drugs with a narrow therapeutic index-like warfarin, lithium, or thyroid hormone-even tiny differences can be risky. The FDA applies the same 80-125% rule here, but scrutinizes the data even more closely. In rare cases, additional studies may be required, but the standard remains unchanged because decades of real-world data show it works.
What Happens If a Generic Fails Bioequivalence?
It doesn’t get approved. Period.
Generic manufacturers submit their data as part of an Abbreviated New Drug Application (ANDA). If the bioequivalence data doesn’t meet the 80-125% threshold, the FDA issues a deficiency letter. The company must fix the formulation-maybe change the coating, the filler, or the manufacturing process-and resubmit.
Many companies run multiple bioequivalence studies before submitting. Some fail. Some succeed. Until 2021, companies only had to report the successful ones. Now, the FDA requires all studies-good and bad-to be submitted. That’s a big shift toward transparency. It means the public can see how many attempts it took to get it right.

When Bioequivalence Isn’t Enough
Not all drugs need blood tests. For medications that work locally-like inhalers for asthma, creams for eczema, or eye drops-the FDA may accept in vitro testing instead. For example, an inhaler might be tested for particle size, spray pattern, and how much drug sticks to the inside of the device. If those match the brand, and the device delivers the same dose, bioequivalence is assumed.
But for drugs meant to enter the bloodstream-oral pills, injections, patches-bioequivalence via PK studies is non-negotiable. The FDA has over 2,000 product-specific guidances that tell manufacturers exactly how to test each drug. These aren’t suggestions. They’re requirements.
The Bigger Picture: Generics Are Safe and Reliable
Despite public skepticism, the science is solid. The FDA’s bioequivalence standard has been in place since 1984, under the Hatch-Waxman Act. Since then, billions of doses of generics have been used safely. Studies comparing brand and generic drugs for conditions like epilepsy, hypertension, and depression show no meaningful difference in outcomes.
Even when patients switch between different generic brands over time-say, from one manufacturer to another-the FDA’s standard ensures consistency. Each version must meet the same 80-125% bioequivalence threshold. So whether you get the generic from Walmart, CVS, or a mail-order pharmacy, the effect is the same.
What’s Next for Bioequivalence?
The FDA is exploring new ways to make testing faster and cheaper without sacrificing safety. For complex drugs-like biologics, topical creams, or inhalers-traditional PK studies are harder to design. The agency is now using computer modeling and simulation to predict how a drug will behave in the body. If the model matches real-world data from the brand-name drug, it may reduce the need for human trials in the future.
But for now, the gold standard remains the same: real people, real blood samples, real data. And the 80-125% rule? Still the backbone of every generic drug approval in the U.S.
Do generic drugs have the same active ingredient as brand-name drugs?
Yes. By law, generics must contain the same active ingredient, in the same strength, and in the same dosage form as the brand-name drug. The difference lies in inactive ingredients-like fillers or coatings-which don’t affect how the drug works.
Why do some people say generics don’t work as well?
Some patients notice differences in pill size, color, or taste when switching to a generic. These are harmless changes in inactive ingredients. Rarely, psychological factors or inconsistent dosing from multiple generic manufacturers can cause perceived differences. But if a generic passes FDA bioequivalence testing, it works the same way clinically.
Can a generic drug be approved without human testing?
Only for drugs that act locally, like nasal sprays, eye drops, or topical creams. For drugs that need to enter the bloodstream, human bioequivalence studies are required. The FDA does not approve oral or injectable generics without testing in people.
How long does it take for the FDA to approve a generic drug?
The standard review time for an ANDA is 10 to 12 months. About 65% of applications get approved on the first try. Many delays happen because of incomplete bioequivalence data or manufacturing issues-not because the drug doesn’t work.
Are generic drugs tested on the same population as brand-name drugs?
No. Generic drugs are tested in healthy volunteers, not patients with the condition. That’s because bioequivalence studies only need to show how the body absorbs the drug-not whether it treats the disease. The safety and effectiveness data from the original brand-name trials still apply to the generic.

Kim Hines
December 16, 2025 AT 12:08The 80-125% range always confused me until I read this. It’s not about how much drug is in the pill-it’s about how your body handles it. That’s actually kind of beautiful when you think about it. Science doesn’t care who made it, just whether it works the same way.
Cassandra Collins
December 18, 2025 AT 05:46lol so you’re telling me the FDA just lets some factory in india pump out pills and says ‘eh close enough’? what if they cut corners? what if the filler is made of crushed chalk and sawdust? i’ve seen the packaging. it looks like a kid drew it. they’re literally just copying the shape and calling it a day. this is how we get poisoned.
Billy Poling
December 20, 2025 AT 00:38It is imperative to underscore that the regulatory framework governing bioequivalence is not merely a statistical artifact but a meticulously calibrated pharmacokinetic benchmark designed to ensure therapeutic equivalence across pharmaceutical formulations. The 90% confidence interval for both Cmax and AUC, constrained within the 80-125% range, is derived from robust, peer-reviewed methodologies that account for inter-individual variability in absorption, distribution, metabolism, and excretion. To conflate this with permissible variation in active pharmaceutical ingredient content is a fundamental misinterpretation of both regulatory intent and scientific principle.
Andrew Sychev
December 20, 2025 AT 22:31They say generics are just as good. But have you ever taken one and felt like your brain was slowly turning to mush? I switched to a generic for my anxiety med and spent three days crying in the shower. The brand? I felt like a human again. Don’t let them fool you. It’s all about profit. They don’t care if you suffer. They just want to sell you a cheaper pill that doesn’t work right.
Arun ana
December 21, 2025 AT 18:37Really appreciate this breakdown! 🙏 I work in pharma logistics in India and see these batches shipped daily. The testing is way stricter than most people think. The labs here have to meet FDA standards too-same equipment, same protocols. It’s not magic, it’s math. And yeah, the 80-125% is about absorption, not dosage. Thanks for clarifying!
Dave Alponvyr
December 22, 2025 AT 03:04So let me get this straight… you’re telling me the FDA doesn’t let generics have 80% of the active ingredient? Wow. I thought they were just cheap knockoffs. Guess I was wrong. Who knew?
Kitty Price
December 22, 2025 AT 04:09That soda analogy? Perfect. 🥤 I always thought generics were like buying a generic brand of cereal-same ingredients, different box. But this? This is like making sure the sugar dissolves at the same rate. Mind blown. Thanks for explaining it so clearly.
Colleen Bigelow
December 23, 2025 AT 18:02They let foreign factories make our medicine? That’s not freedom, that’s national suicide. Our doctors, our scientists, our lives-reduced to a cost-cutting spreadsheet. And now they want us to trust a pill made by some guy in Bangalore who speaks three languages and doesn’t even know what ‘bioequivalence’ means? This is how empires fall.
Randolph Rickman
December 23, 2025 AT 21:17This is the kind of info we need more of. People panic about generics because they don’t understand the science, not because it’s unsafe. The FDA’s system has kept millions of people healthy for decades. If your generic works, it works. If it doesn’t, it didn’t pass. Simple. No drama. Just good science.
sue spark
December 24, 2025 AT 17:19So if two generics have the same active ingredient but different fillers and one works better for me than the other is that normal or should I be worried